BICOM optima®
Certified medical device Class IIa
Clinical study report
Allergic rhino-conjunctivitis with BICOM optima®


Post Market Clinical follow-up study on the performance and safety of BICOM optima® / BICOM optima® mobil devices for bioresonance treatment in patients with allergic rhino-conjunctivitis
Synopsis
This clinical study report describes the design, execution and statistical analysis of the BICOM optima/PMCF study on the treatment of allergic rhino-conjunctivitis with BICOM optima® devices. The report covers the period 25.01.2021 until 10.01.2022 (LPLV). This final report is based on the final monitored data of the study after data-analysis.
The full study report in English will soon be available for download from the German Clinical Trials Registry using the registration number below.
Further Information
For more information on the use of the BICOM optima® devices, please contact the nearest international representative.
Clinical Study Report
- Title of the study: Prospective, multi-center, single-arm, open-label, observational study for the evaluation of performance and safety of the BICOM optima® / BICOM optima® mobil device for bioresonance treatment in patients with allergic rhino-conjunctivitis
- Study centre: 9 study sites in Germany, 8 collected patient data.
- Study period: Date first patient enrolled: 25.01.2021; date last patient enrolled: 14.10.2021
- Registration: DRKS00024523
- Phase: Post-market; device is used according to IFU
- Investigational device: BICOM optima® / BICOM optima® mobil (B32, B34 and BM34).
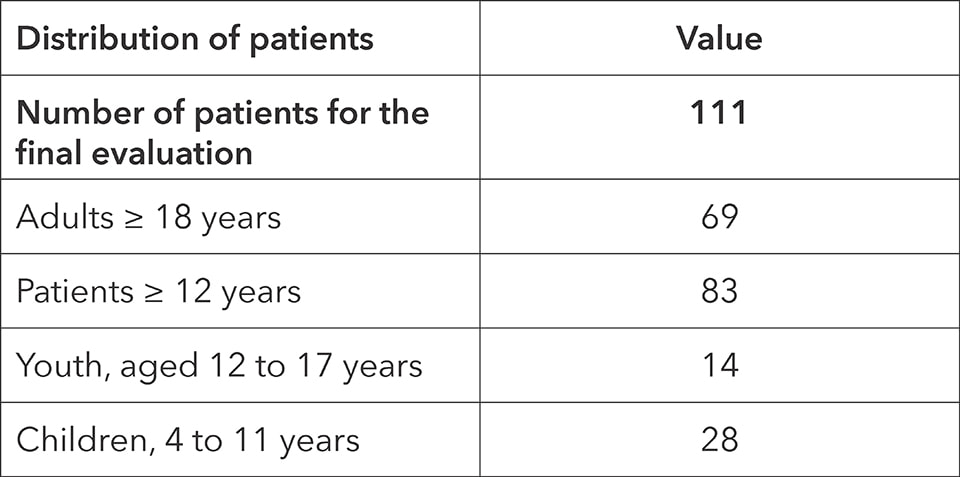
- Patients: 111 (28 children from 4 to 10 years, 14 youth from 12 to 17 years and 69 adults, 18 years and older)
- Patient population: Patients 4 years and older diagnosed with allergic rhino-conjunctivitis caused by pollen (e.g. tree, grass), house dust mite (HDM) and animal hair allergy. Patients with mild to moderate allergic rhino-conjunctivitis were treated according to the clinical routine and in accordance with the current IFU.
- Coordinating principal investigator: Dr. med. Jürgen Hennecke, Fichtestraße 29, 52078 Aachen, Germany
This study was conducted in accordance with ISO 14155 that ensured adherence to Good Clinical Practices and protection of the subjects, as required by the directives in operation at the time.
1 Rationale for the study
BICOM® bioresonance therapy is a therapy technique that has been part of the field of complementary medicine for over thirty years. It proposes that low energy electromagnetic waves can be used to treat human illness. The theory is based on established scientific findings of particle physics that each type of matter has its own electromagnetic field. Well-known medical diagnostic systems like EEG, ECG, EMG, MRT and MEG already use external, technically generated electromagnetic fields for diagnostic and therapeutic purposes.
1.1 Basic functioning
The principle of electromagnetic fields may also be applied to every cell or organ of the human body. The BICOM® device picks up these bioelectromagnetic fields, alters them and returns them to the patients to achieve a therapeutic effect. For this purpose, the patient is connected to the BICOM® device. Patient and device form a so-called bio-cybernetic control-circuit. The device picks up the patient´s oscillations and processes them: they may be neutralized altered, amplified or otherwise conditioned and then returned to the patient.
1.2 Scientific stance
Several clinical studies suggest that bioresonance therapy is particularly effective in the treatment of allergic rhinitis and allergic conjunctivitis (hay fever). Significant treatment success has been demonstrated in these studies using different research approaches. During the therapy, among other things, the electromagnetic fields of the allergens are altered and returned to the patient to achieve therapeutic success. Results suggest that bioresonance therapy reduces allergic symptoms and successfully treats allergies, where “therapy” in this context means: freedom from allergic symptoms and no reoccurrence of symptoms within six months of the end of the treatment. The treatment is given weekly and takes three to twenty sessions.
The best-known other type of treatment for long-term elimination of allergies is allergen immunotherapy (AIT), commonly known as “desensitization”. This usually takes three to five years and can be quite costly and restrictive for the patient. Bioresonance therapy is much faster and cheaper. In addition, studies have shown that the bioresonance method reduces or even eliminates the need for symptom-relieving allergy medications. The elimination of medication often has multiple health benefits for the patient.
Several clinical studies have already shown the efficiency of bioresonance therapy devices and the sustainable success of bioresonance therapy. These studies were however mostly performed outside the EU and at a time where international clinical standards like the GCP (Good Clinical Practice) and ISO 14155 norm did not exist. This Post-Market Clinical Follow-up study (PMCF), was conducted according to current GCP and ISO 14155 requirements, and is intended to confirm the results of previous studies.
2 Legal framework
This study gathered clinical data on BICOM® devices manufactured by REGUMED®, Regulative Medizintechnik GmbH. The aim was to test the performance and safety of the BICOM optima® / BICOM optima® mobil devices (B32, B34 and BM34). The devices carried a CE-mark and were used according to the instructions for use (IFU).
The study aimed to collect clinical data for the BICOM® devices in use. The devices are medical devices (risk class IIa) for professional use. They were developed exclusively for use by trained, licensed doctors, state-approved naturopaths or trained medical professionals under their supervision.
The commissioned research institute conducted the study in accordance with the professional code of conduct for doctors in Germany and was advised by the Ethics Committee of the North Rhine Medical Association (Ethikkommission Ärztekammer Nordrhein).
2.1 Information and data protection
All patients consented to participate in writing before the start of the study. Among other things, the patients were informed about data protection, the use of their medical data and their right to withdraw from the study at any time without specification of reasons. In addition, the applicable data protection regulations, in particular the EU General Data Protection Regulation (GDPR) was applied.
2.2 Monitoring
The study was monitored in accordance with the Declaration of Helsinki, ISO 14155:2020, the Clinical Investigational Protocol (CIP), the signed agreement between REGUMED® and the research institute as well as national regulations.
2.3 Adaptation for general publication
The study has been summarized by REGUMED® for the purpose of general publication in this document.
3 BICOM® bioresonance therapy
BICOM® bioresonance therapy is a method from the field of complementary medicine. Electromagnetic interactions play as much a role in cell communication and the transmission of information, as electrical processes at the receptor proteins and bio membranes. Specific electromagnetic wave patterns act as carriers of information. These wave patterns can be modulated by the BICOM® devices to eliminate disturbing or stressful information in the body. The aim is to restore the free flow of healing information (cell-communication) and thus to stimulate the organism’s self-regulation and the self-healing powers of the body.
The therapy uses individual patient-information as well as the information of native and digitalized substances. Discrepancies with findings from conventional medicine arise from these different points of view. While BICOM® bioresonance therapy focuses on information and principles from quantum-physics, the approach of conventional medicine is still based on the mechanistic-deterministic worldview (Newton).
BICOM® devices are recommended for the treatment of mild to moderate allergies and allergy-related diseases or complications. They focus on allergic rhino-conjunctivitis and are suitable for treating children and adults alike.
3.1 Allergic rhino-conjunctivitis
Allergic rhinitis is a chronic inflammation of the nasal mucosa triggered by an immune response of the body as a result of a hypersensitive allergic reaction. Symptoms of allergic rhinitis include a runny nose, nasal congestion, nasal itching, and repeated sneezing. It is often accompanied by allergic conjunctivitis with symptoms that can include itchy, red, watery, and/or swollen eyes.
Studies suggest a wide prevalence of allergies within the German population.
According to this research, nearly every third adult will be diagnosed with an allergy by a doctor at some point in their lives. The most common diseases are rhino-conjunctivitis (around 15%), followed by asthma and contact eczema (around 8%), food allergies (around 5%) and atopic dermatitis, urticaria as well as insect venom allergies (around 3% each). Studies for children find a prevalence of neurodermatitis (almost 13%), hay fever (11%), bronchial asthma (6%), and allergic contact dermatitis (nearly 3%).
Due to the non-life-threatening nature of symptoms, allergies are often considered to be unimportant or minor diseases. They are, however, increasingly recognized as having a major effect on quality of life, emotional well-being, sleep, daily activities and productivity when poorly controlled.
Restrictions are often severe, with patients unable to find a basic therapy.
3.2 Treatment prospects
The first form of treatment for allergies is typically allergen avoidance. Patients are advised to limit exposure to the relevant allergens. After that allergies are usually treated with antihistamines, corticosteroids, leukotriene antagonists and Beta2-adrenergic agonists. Allergen immunotherapy, commonly known as “desensitization”, is the best-known other treatment method for eliminating allergies.
Desensitization is the practice of gradually introducing allergens to the patient in larger and larger amounts in order to gradually accustom the body to the allergen and reduce the allergic symptoms. Treatment take three to five years and and can be very costly. It also involves a lot of effort on the part of the patient. Therefore, only a few patients turn to this treatment option. In addition, patients are at risk of suffering severe side effects, including anaphylaxis.
BICOM® bioresonance therapy offers a better method of treatment. The method has been used for decades to treat allergies and their associated symptoms and has fewer risks and side effects. In addition, studies have shown that the use of allergy medications can be reduced or even stopped through the use of bioresonance therapy. The required number of treatment-sessions depends on the severity of the allergy and generally ranges between three to twenty sessions at weekly intervals.
Thus, the duration of treatment is significantly shorter than with desensitization. A session lasts no longer than an hour and is administered automatically by the BICOM® bioresonance device after having been programmed by a doctor, practitioner or medical trained personnel. Good education of the doctor or naturopathic practitioner is crucial for the success of the treatment.
The BICOM® devices may only be operated by medically trained personnel.
4 The Post-Market Clinical Follow-up study
The study investigated whether the symptoms of rhino-conjunctivitis improved with routine treatment using the BICOM® method. The patient sample was representative of the average patient population, including children from the age of 4 years. There were only a few exclusion criteria.
4.1 Evaluation of the decrease in symptoms
The main objective was to measure the change in the patients´ weekly symptoms. For this purpose, the patients received a questionnaire with six listed symptoms. They were asked to choose a score ranging from zero (no symptoms at all) to three. The symptoms were nasal irritation, sneezing, runny nose, stuffy nose, itchy/red eyes and watery/swollen eyes.
An improvement of one point was considered to be a success of the treatment. In addition, data was collected on quality of life, need for medication and acute symptoms. This was measured by three additional scores.
4.2 Evaluation of adverse effects
To evaluate the safety of BICOM® bioresonance therapy, patients were asked about any adverse effects. These could be triggered either by the device or the treatment. Adverse effects and serious adverse effects were included in the questionnaire from the beginning as part of the study. In the case of serious adverse effects the treatment was discontinued and the incident was noted.
Doctors and therapists working with the BICOM® bioresonance method have long been familiar with the phenomenon of a short-term worsening of symptoms during treatment, the so called initial aggravation. This is a known effect of the therapy and these symptoms are considered desirable as they are an indicator that the therapy is beginning to work. They represent activation of the immune system and should not be suppressed. They can however be alleviated by medication. Severe cases of initial aggravation are an exception. In these cases, the decision is at the discretion of the doctor or naturopathic practitioner.
The severity of the initial reaction depends on the severity and type of disease being treated. Initial worsening was included in the questionnaire as an adverse effect. For general information on contraindications, warnings and side effects, consult a doctor or naturopathic practitioner or refer to the instructions for use (IFU) of the BICOM® devices.
4.3 Method of collecting data
Patient data was always collected before the start of every treatment session, including the first. An additional questionnaire was conducted within two weeks after the end of the last treatment (follow-up). Only treatments that lasted three sessions or longer were included in this study. If a patient required more than eight treatment sessions, only the first eight sessions were included, or the first fifteen weeks of treatment, whichever came first.
4.4 The study at a glance
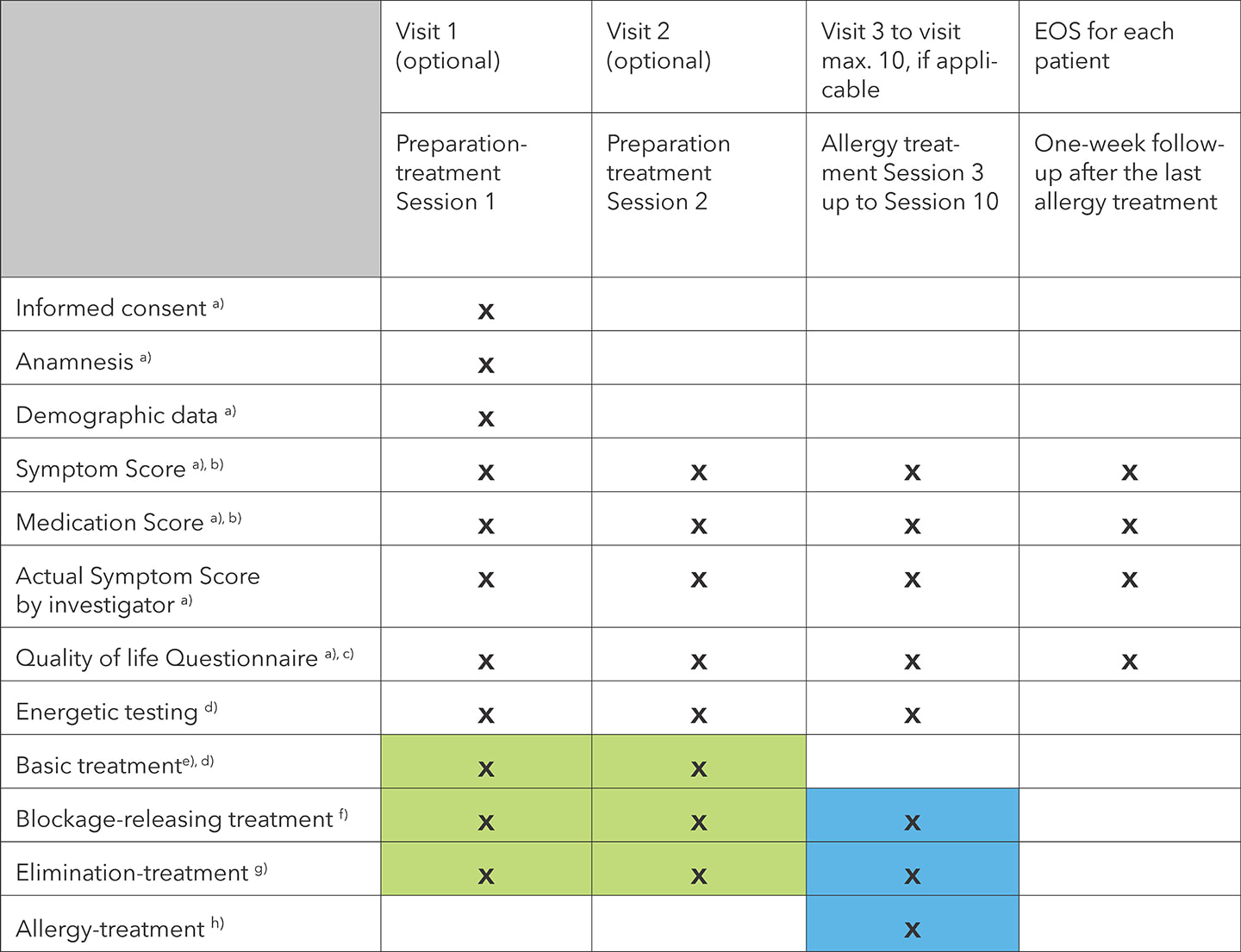
- Data from patients who did not receive preparation-treatment was collected from the first allergy treatment session.
- This score asked about the most severe symptoms and their duration in days, as well as medication including the duration of medication in days.
- This score assessed the impact of rhino-conjunctivitis on quality of life.
- This test is part of the standard procedure when using BICOM® bioresonance devices.
- A basic therapy program or sequence aims to prepare the patient for allergy treatment.
- Up to three blockage-releasing programs or sequences could be used after bioenergetic testing.
- Up to three elimination programs or sequences could be used after bioenergetic testing.
- Allergy treatment involved the use of allergy therapy programs as well as supportive symptom-related programs or sequences.
- Adverse device effects (ADE) and serious adverse device effects (SADE) were recorded from the first treatment session.
Note: Before each treatment session, the symptoms, the medication, and the quality of life scores were recorded. This also applied to the two preparation treatments with “basic treatment”, “blockage-releasing treatment” and “elimination treatment”.
5 Results
Data from eight study-sites was used in the evaluation. 111 patients were part of the study. The first subject was surveyed on January 25th 2021 and the last patient was surveyed on January 10th 2022.
On average, patients had suffered from rhino-conjunctivitis for 13.8 years. The main triggers were pollen, followed by house dust mite and animal hair. The majority of patients had a combination of triggers.
5.1 Alleviation of symptoms
The main objective of the evaluation was to evaluate changes in symptoms. Patients regularly completed a questionnaire. They were asked about nasal irritation, sneezing, runny nose, nasal congestion, itchy/red eyes and watery/swollen eyes. The patients could choose between a severity of 0 (no symptoms) to 3.
The weekly Symptom Score (wSS) represents the severity of symptoms during the previous week. The lower the wSS-value, the lesser the patient’s symptoms were. The lower the score, the lower were the symptoms. The maximum symptom-score was 18 (six times three). The minimum symptom-score was 0.
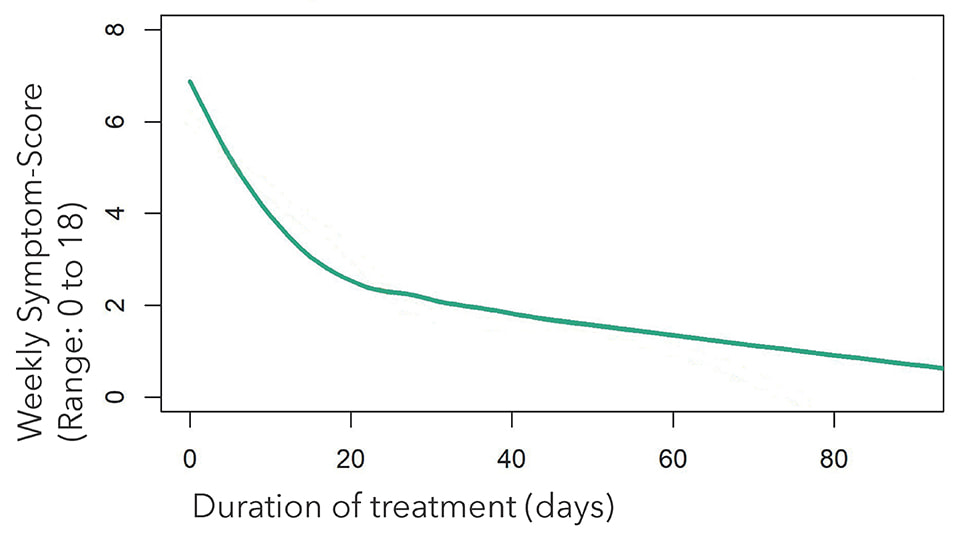
The baseline symptom score was recorded at the start of the therapy. Data was also collected during treatment sessions and once after the end of the last session (follow-up). From this, an average value was calculated from the weekly Symptom Scores and measured against the starting baseline score. The endpoint of recovery was reached on average after 4.4 sessions.
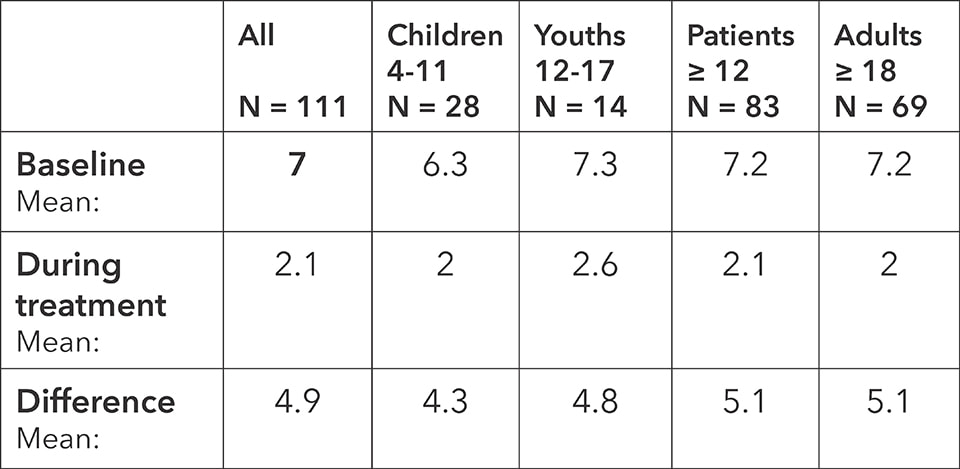
Weekly Symptom Score: baseline, average during the treatment and difference
The symptoms decreased on average from 7 to 2.1 points in all patients, reflecting a clinically and statistically significant improvement for the whole sample. The absolute change in score, which is 4.9, is clearly above the target reduction of 1 point, which was set at the beginning of the study to measure efficacy of the treatment.
For all age groups, the results are clinically and statistically highly significant independently of each other. reductions in symptoms are similarly independently highly statistically significant. The study thus clearly demonstrates the reduction of symptoms through treatment with BICOM® bioresonance.
5.2 Improvement in quality of life
The study also evaluated the change in quality of life as perceived by the patient. The questionnaire for this was composed of six items: patients were asked to fill in scores about their restrictions in wellbeing, sleep, everyday activities, sports activities, school or professional activities and social contacts. A scale ranging from 0 to 4 was used for this, with 0 meaning no limitations. In this way, the gravest of limitations would be a score of 24.
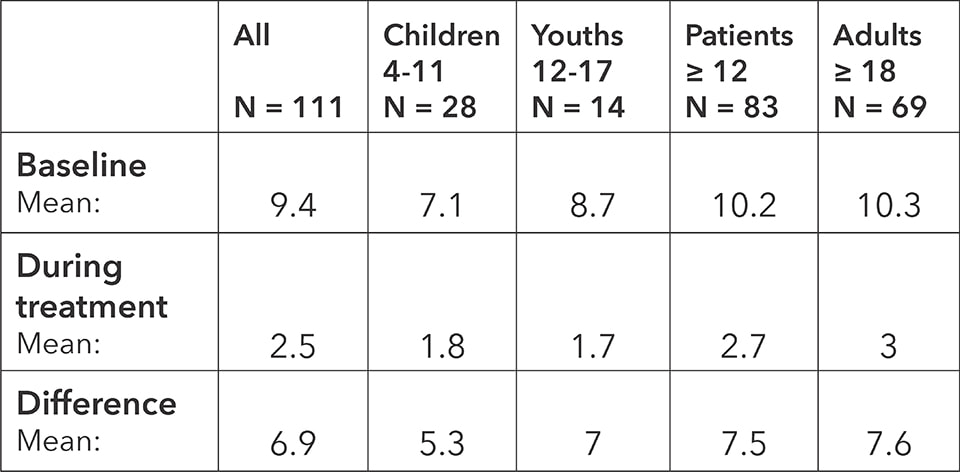
Quality of Life Score: baseline, average during the treatment and difference
The score shows a significant decrease in restrictions on the quality of life from a score of 9.4 to 2.5 points for all age groups. The difference of 6.9 points represents an improvement of almost 75 percent compared to the baseline. Improvements are seen in all age groups. The study thus demonstrates a significant increase in the quality of life for patients with rhino-conjunctivitis when treated with BICOM® bioresonance.
5.3 Changes in the need for medication
The need for symptom easing medication was another point of analysis in this study. For this purpose, the patients filled out a questionnaire on their need for medication as a score on a weekly basis. A decrease in the need for medication is consider a success of BICOM® bioresonance therapy. The medication score assessed the use of antihistamines for ingestion or in the form of eye drops, nasal sprays, intranasal glucocorticoids with/without antihistamines and glucocorticoids with/without intranasal glucocorticoids or with/without antihistamines.
The study showed that most patients did not use conventional medications before the start of the first session and that this did not change during treatment. According to the research institute commissioned, the data on a possible reduction in the need for medication is therefore not conclusive. Further evaluation, for example by reviewing the mean values with regard to possible improvements, must therefore be omitted.
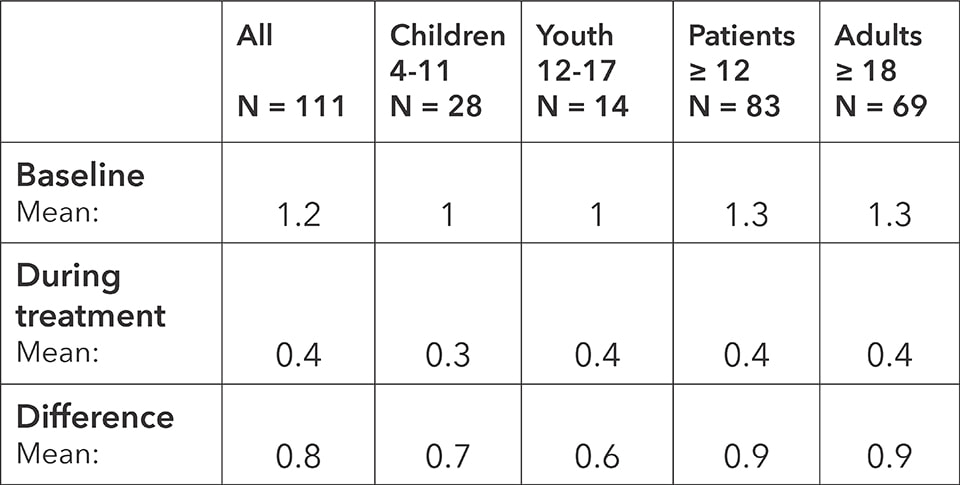
5.4 Improvement of acute symptoms
Before every treatment session and one week after the last allergy treatment (follow up), the acute symptoms were surveyed by the examining doctor or therapist. Low values represent fewer and or weaker symptoms.
Acute symptoms: baseline, average during the treatment and difference
The study shows a significant improvement in acute symptoms across all age groups during BICOM® bioresonance treatment. On average, symptoms improved 66 percent from the baseline score, from 1.2 to 0.4. The results were similar for all age groups. The study thus demonstrates the improvement of acute symptoms for patients with rhino-conjunctivitis when treated with BICOM® bioresonance therapy.
5.5 Adverse effects
Six patients experienced adverse effects. There were however no serious complications and no serious adverse effects. All cases were mild to moderate and all patients recovered. One patient, in consultation with the doctor, decided not to continue treatment due to the side effects. The study found no indications in which to change the existing positive risk-benefit assessment.
5.6 Final conclusion
The result of this study shows that the treatment of mild to moderate rhino-conjunctivitis with BICOM® bioresonance therapy leads to a significant improvement in symptoms and quality of life. The study demonstrates the efficacy of the treatment and the benefit to the patient. In addition, BICOM® devices are very safe. No serious adverse effects occurred during the course of the study, which ran for more than a year.
All rights reserved, Published August 8, 2022
Any questions?
Arrange a consultation with our international BICOM® experts
They are available to answer all your questions and will be happy to advise you personally and individually.